
IN THE FACE OF A PANDEMIC
HICCC Researchers Address COVID-19
Cracking Down on COVID-19
Faces of the Frontline
CONNECTING THE DOTS IN CANCER
Engineering + Cancer
Dentistry + Cancer
Public Health + Cancer
RESEARCHERS ON THE CUTTING EDGE
Hitting Pancreatic Cancer Where it Hurts
Commanding Killer T Cells
Election to National Academies
MAKING A DIFFERENCE
A “CURE” for Budding Scientists
Newfound Hope with Cell TherapyVelocity Goes Virtual
Unicorns in the Treatment Rooms
ACKNOWLEDGMENTS
CONTACT US

IN THE FACE OF A PANDEMIC
HICCC Researchers Address COVID-19
Cracking Down on COVID-19
Faces of the Frontline
CONNECTING THE DOTS IN CANCER
Engineering + Cancer
Dentistry + Cancer
Public Health + Cancer
RESEARCHERS ON THE CUTTING EDGE
Hitting Pancreatic Cancer Where it Hurts
Commanding Killer T Cells
Election to National Academies
MAKING A DIFFERENCE
A “CURE” for Budding Scientists
Newfound Hope with Cell TherapyVelocity Goes Virtual
Unicorns in the Treatment Rooms
ACKNOWLEDGMENTS
CONTACT US
Columbia University biologist Kenneth Olive, PhD, isn’t afraid to tackle a hard problem. He has dedicated his life to finding a cure for pancreatic cancer, one of the toughest types of cancer to treat. Ninety percent of patients in the U.S. diagnosed with this deadly disease will die within just five years, making it the cancer with the lowest overall survival rate.
“What it comes down to is that pancreatic cancer doesn’t respond well to existing drugs,” says Dr. Olive, associate professor of medicine and member of the Precision Oncology and Systems Biology research program at the Herbert Irving Comprehensive Cancer Center (HICCC). “We take the entire arsenal that has been deployed against other cancers, test it on pancreatic cancer, and there is little effect.”
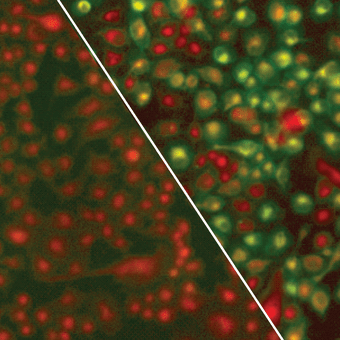
Green fluorescence demonstrates lipid oxidation in pancreatic cancer cells deprived of cysteine. This leads to rapid, tumor-selective cell death.
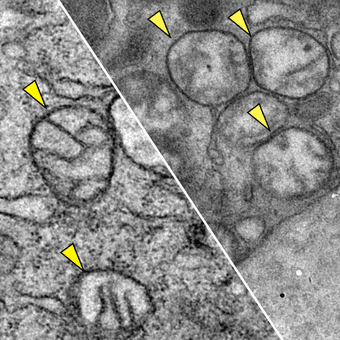
Transmission electron microscopy images of mitochondria in the pancreatic tumor cells of two mice from the Olive lab study on cysteine. Left: healthy, normal mitochondria (small parts of a cell that provide energy) in a mouse that was untreated; Right: shows mitochondria that have been damaged by ROS in the pancreatic tumor of a mouse that was treated with cysteinase. Yellow triangles indicate the mitochondria.
Cover photo:
Kenneth Olive, PhD
RESEARCHERS ON THE CUTTING EDGE
HITTING PANCREATIC CANCER WHERE IT HURTS
Hitting
Pancreatic
Cancer
Where It Hurts
Hitting
Pancreatic
Cancer
Where It Hurts
Dr. Olive's Laboratory Works on Finding New Strategies For Treatment
Columbia University biologist Kenneth Olive, PhD, isn’t afraid to tackle a hard problem. He has dedicated his life to finding a cure for pancreatic cancer, one of the toughest types of cancer to treat. Ninety percent of patients in the U.S. diagnosed with this deadly disease will die within just five years, making it the cancer with the lowest overall survival rate.
“What it comes down to is that pancreatic cancer doesn’t respond well to existing drugs,” says Dr. Olive, associate professor of medicine and member of the Precision Oncology and Systems Biology research program at the Herbert Irving Comprehensive Cancer Center (HICCC). “We take the entire arsenal that has been deployed against other cancers, test it on pancreatic cancer, and there is little effect.”

Kenneth Olive, PhD
“When I started my career 20 years ago, malignant melanoma was almost uniformly lethal. But over the years, a combined immunotherapy approach has led to frequent major responses to the disease. That’s a really great archetype to reach for pancreatic cancer, and we’re beginning to see examples of drugs or drug combinations that produce more frequent minor responses. So I’m optimistic.”
Green fluorescence demonstrates lipid oxidation in pancreatic cancer cells deprived of cysteine. This leads to rapid, tumor-selective cell death.
Transmission electron microscopy images of mitochondria in the pancreatic tumor cells of two mice from the Olive lab study on cysteine. Left: healthy, normal mitochondria (small parts of a cell that provide energy) in a mouse that was untreated; Right: shows mitochondria that have been damaged by ROS in the pancreatic tumor of a mouse that was treated with cysteinase. Yellow triangles indicate the mitochondria.
RESEARCHERS ON THE
CUTTING EDGE
CUTTING EDGE
HITTING PANCREATIC CANCER
WHERE IT HURTS

“When I started my career 20 years ago, malignant melanoma was almost uniformly lethal. But over the years, a combined immunotherapy approach has led to frequent major responses to the disease. That’s a really great archetype to reach for pancreatic cancer, and we’re beginning to see examples of drugs or drug combinations that produce more frequent minor responses. So I’m optimistic.”
His laboratory works on finding new strategies for treatment that target aspects of biology needed by pancreatic cancer to survive. In other words, Dr. Olive and his colleagues look for an Achilles’ heel—called a targetable critical dependency—and see how well a certain drug or combination of drugs works to exploit that weakness.
Recently, they discovered that a drug in development for a rare kidney stone disease acts on one of these targetable critical dependencies for pancreatic cancer. Cancer cells have a detoxification system that allows them to get rid of reactive oxygen species (ROS), which left uncontrolled, will wreak havoc on DNA and key proteins. When tumors grow, they create an excess of ROS, and it needs to be cleared by the detoxification system.
The researchers wondered if dismantling this detoxification system and letting ROS run wild in tumor cells would prove effective against pancreatic cancer. They decided to inhibit a particular transporter that brings an amino acid called cysteine — a key resource needed for the detox process—inside the cell. To test their hunch, Dr. Olive and his colleagues genetically engineered mice in which the transporter could be suddenly deleted and found that this caused their tumors to stop growing or regress.
“Of course, we’re not going to genetically engineer patients, so we needed a drug to do the same thing,” he says. “We encountered a paper reporting on an artificial enzyme that could break down cysteine in the blood. We thought, this seems appropriate—fortuitous, in fact.”
They obtained the enzyme, which is being developed for the treatment of cystinuria, a genetic disorder that leads to high levels of cysteine in the urine. When given to mice with pancreatic tumors, the cancer cells began to die. This work, helmed by Dr. Olive’s former graduate student Michael A. Badgley, PhD, was published by Science in April.
While his research continued during the pandemic, Dr. Olive took on another challenging role. He helped launch an organization, Columbia Researchers Against COVID-19, which connected volunteers with hospitals and labs that needed help responding to the pandemic. Columbia students, postdocs, and technicians forced to stay out of the labs during lockdown gave their free time to aid local hospitals and COVID-19 research efforts.
“We ended up with 750 volunteers who wanted to contribute. The largest project was a deployment of over 150 volunteers to the NewYork-Presbyterian Hospitals at the height of the pandemic,” says Dr. Olive, who also directs the Oncology Precision Therapeutics and Imaging Core at the HICCC. “We staffed four hospitals for three shifts, 24 hours a day, seven days a week providing manual labor, folding scrubs, and handing out meals.”
Since the university’s reopening, his lab has kept busy while exercising safety precautions like rotating shifts and social distancing. His focus has returned to solving the puzzle of pancreatic cancer, and despite the difficulty it poses, Dr. Olive remains hopeful that survival rates will improve. He gives the example of malignant melanoma, a once-lethal cancer that has an improved outlook today due to a wave of new treatments.
“When I started my career 20 years ago, malignant melanoma was almost uniformly lethal. But over the years, a combined immunotherapy approach has led to frequent major responses to the disease,” says Dr. Olive. “That’s a really great archetype to reach for pancreatic cancer, and we’re beginning to see examples of drugs or drug combinations that produce more frequent minor responses. So I’m optimistic.”
His laboratory works on finding new strategies for treatment that target aspects of biology needed by pancreatic cancer to survive. In other words, Dr. Olive and his colleagues look for an Achilles’ heel—called a targetable critical dependency—and see how well a certain drug or combination of drugs works to exploit that weakness.
Recently, they discovered that a drug in development for a rare kidney stone disease acts on one of these targetable critical dependencies for pancreatic cancer. Cancer cells have a detoxification system that allows them to get rid of reactive oxygen species (ROS), which left uncontrolled, will wreak havoc on DNA and key proteins. When tumors grow, they create an excess of ROS, and it needs to be cleared by the detoxification system.
The researchers wondered if dismantling this detoxification system and letting ROS run wild in tumor cells would prove effective against pancreatic cancer. They decided to inhibit a particular transporter that brings an amino acid called cysteine—a key resource needed for the detox process—inside the cell. To test their hunch, Dr. Olive and his colleagues genetically engineered mice in which the transporter could be suddenly deleted and found that this caused their tumors to stop growing or regress.
“Of course, we’re not going to genetically engineer patients, so we needed a drug to do the same thing,” he says. “We encountered a paper reporting on an artificial enzyme that could break down cysteine in the blood. We thought, this seems appropriate—fortuitous, in fact.”
They obtained the enzyme, which is being developed for the treatment of cystinuria, a genetic disorder that leads to high levels of cysteine in the urine. When given to mice with pancreatic tumors, the cancer cells began to die. This work, helmed by Dr. Olive’s former graduate student Michael A. Badgley, PhD, was published by Science in April.
Hitting
Pancreatic
Cancer
Where It Hurts
Dr. Olive's Laboratory Works on Finding New Strategies For Treatment
While his research continued during the pandemic, Dr. Olive took on another challenging role. He helped launch an organization, Columbia Researchers Against COVID-19, which connected volunteers with hospitals and labs that needed help responding to the pandemic. Columbia students, postdocs, and technicians forced to stay out of the labs during lockdown gave their free time to aid local hospitals and COVID-19 research efforts.
“We ended up with 750 volunteers who wanted to contribute. The largest project was a deployment of over 150 volunteers to the NewYork-Presbyterian Hospitals at the height of the pandemic,” says Dr. Olive, who also directs the Oncology Precision Therapeutics and Imaging Core at the HICCC. “We staffed four hospitals for three shifts, 24 hours a day, seven days a week providing manual labor, folding scrubs, and handing out meals.”
Since the university’s reopening, his lab has kept busy while exercising safety precautions like rotating shifts and social distancing. His focus has returned to solving the puzzle of pancreatic cancer, and despite the difficulty it poses, Dr. Olive remains hopeful that survival rates will improve. He gives the example of malignant melanoma, a once-lethal cancer that has an improved outlook today due to a wave of new treatments.
“When I started my career 20 years ago, malignant melanoma was almost uniformly lethal. But over the years, a combined immunotherapy approach has led to frequent major responses to the disease,” says Dr. Olive. “That’s a really great archetype to reach for pancreatic cancer, and we’re beginning to see examples of drugs or drug combinations that produce more frequent minor responses. So I’m optimistic.”
© Columbia University Irving Medical Center,
Herbert Irving Comprehensive Cancer Center,
New York, NY.
Credit and Cast
Download Print-friendly
HICCC Annual Report 2020

