
IN THE FACE OF A PANDEMIC
HICCC Researchers Address COVID-19
Cracking Down on COVID-19
Faces of the Frontline
CONNECTING THE DOTS IN CANCER
Engineering + Cancer
Dentistry + Cancer
Public Health + Cancer
RESEARCHERS ON THE CUTTING EDGE
Hitting Pancreatic Cancer Where it Hurts
Commanding Killer T Cells
Election to National Academies
MAKING A DIFFERENCE
A “CURE” for Budding Scientists
Newfound Hope with Cell TherapyVelocity Goes Virtual
Unicorns in the Treatment Rooms
ACKNOWLEDGMENTS
CONTACT US

IN THE FACE OF A PANDEMIC
HICCC Researchers Address COVID-19
Cracking Down on COVID-19
Faces of the Frontline
CONNECTING THE DOTS IN CANCER
Engineering + Cancer
Dentistry + Cancer
Public Health + Cancer
RESEARCHERS ON THE CUTTING EDGE
Hitting Pancreatic Cancer Where it Hurts
Commanding Killer T Cells
Election to National Academies
MAKING A DIFFERENCE
A “CURE” for Budding Scientists
Newfound Hope with Cell TherapyVelocity Goes Virtual
Unicorns in the Treatment Rooms
ACKNOWLEDGMENTS
CONTACT US
Engineering+ Cancer
+ Cancer
An Unlikely Match:Bacteria + Immunotherapy
An Unlikely Match:
Bacteria + Immunotherapy
When it comes to having a successful research collaboration, human chemistry plays an equally important role as the science itself.
This stands true for frequent collaborators Nicholas Arpaia, PhD, Tal Danino, PhD, and their joint PhD student, Sreyan Chowdhury. In 2019, the trio’s Nature Medicine paper grabbed wide media attention and accolades from the scientific community for its innovation and incredible promise in the cancer immunotherapy space.
In fact, it was their co-mentee and student, Chowdhury, who got this dream team together in the first place.
Inspired by the explosion of cancer immunotherapy studies, Chowdhury, a PhD student in the Integrated Program at Columbia’s Vagelos College of Physicians & Surgeons, began brainstorming ways to couple his interest in synthetic biology with cancer immunotherapy. At the time, Chowdhury had been rotating in the Danino lab, and Dr. Danino, associate professor of biomedical engineering and member of the Herbert Irving Comprehensive Cancer Center (HICCC), had been investigating strains of bacteria for a programmable delivery system to kill cancer cells.
Center graphic:
A plasmid map of ‘pSC01’ which encodes the synchronized lysis circuit that enables bacteria to lyse and release the researchers’ engineered immunotherapeutic payload within the tumor.
Lower Left Image:
Histological section of a lymphoma tumor removed from a mouse showing tumor cells (round, pink) and bacteria (tiny rod-shaped objects in the white space).
“I wondered whether we could use these programmable bacteria to home to tumors, grow, and release waves of potent immunotherapeutics exclusively within the cores of tumors,” says Chowdhury. “The hope was that we could sustainably deliver high-dose immunotherapy within the tumor, while preventing off-target toxicity and side effects. For this, Tal and I both knew we needed an expert in immunology.”
The beauty of Columbia’s Integrated Program in Cellular, Molecular, and Biomedical Studies (CMBS), is that it allows for graduate students in their first year to rotate in any Columbia lab involved in biomedicine broadly, and that’s precisely what Chowdhury did. A module of the course was taught by Dr. Arpaia, assistant professor of microbiology and immunology and member of the HICCC. Chowdhury began learning more about Dr. Arpaia’s research, including his graduate work in understanding immune factors governing virulence of pathogens and his postdoctoral work on the diverse roles of regulatory T cells in health and disease.
“Inspired by Nick’s papers and lectures in immunology, I brought up the idea of setting up a meeting between the three of us. Tal is super supportive of his trainees forging new research directions for the lab so he instantly agreed,” says Chowdhury.
The three have since formed a tightly knit research team, with their eyes set on cutting-edge cancer research. Both Drs. Arpaia and Danino lean to out-of-the-box ideas that meld bacterial cancer therapy and immunotherapy in a way to increase its efficacy as a therapeutic and also enhance its safety.
In the Nature Medicine paper, the researchers demonstrated a novel engineered system to deliver immunotherapy from bacteria, which is priming the patient’s own immune system to fight the cancer. In a mouse model of lymphoma, the therapy led not only to complete regression of treated tumors, but also demonstrated it could prime the immune system to seek and treat distant untreated tumors.
The team recently developed engineered probiotics to safely deliver immunotherapies within tumors, work that was published February 2020 in Science Translational Medicine. These include nanobodies against two proven therapeutic targets—PD-L1 and CTLA-4. Through this platform, drugs are continuously released by bacteria and continue to attack the tumor after just one dose, facilitating an immune response that ultimately results in tumor regression. The versatile probiotic system, say the researchers, can also be used to deliver multiple immunotherapies simultaneously, enabling the release of effective therapeutic combinations within the tumor for more difficult-to-treat cancers like colorectal cancer.
While true for many research fields, investigating a complex problem like cancer requires expertise from multiple disciplines. Says Dr. Arpaia, “In order to have a successful multidisciplinary collaboration, you have to have trainees who are committed to doing research in a truly interdisciplinary fashion.”
Trust plays a big role.
“I don’t know everything Tal knows about synthetic biology, and I’m not going to. And Tal isn’t going to know everything I know about immunology,” says Dr. Arpaia. “Our collaboration is us agreeably trusting each other for our own expertise. And, we’re jointly making sure that we have our bases covered but also explaining to each other the cool parts about our fields.”
The team is working on optimizing their bacteria-based cancer immunotherapy to eventually test in the clinic, and have had conversations about therapeutic dosing and toxicity with Gary Schwartz, MD, deputy director of the HICCC, an oncologist, and visionary in targeted cancer therapy. At the end of the day, adds Dr. Arpaia, “We are all equally committed to doing impactful science the best way we can.”
“I’m so happy that Nick and Tal are writing and winning multiple grants together and involving more members of both labs on collaborative projects,” says Chowdhury. “They’re pretty close friends now, and I’m happy to have been the catalyst.”
Cover photo:
Sreyan Chowdhury (left), Tal Danino, PhD (middle), and Nick Arpaia (right) PhD.
CONNECTING THE DOTS IN CANCER
Engineering
+ CanceR
Engineering
+ Cancer
An Unlikely Match:Bacteria + Immunotherapy
An Unlikely Match:
Bacteria + Immunotherapy
When it comes to having a successful research collaboration, human chemistry plays an equally important role as the science itself.
This stands true for frequent collaborators Nicholas Arpaia, PhD, Tal Danino, PhD, and their joint PhD student, Sreyan Chowdhury. In 2019, the trio’s Nature Medicine paper grabbed wide media attention and accolades from the scientific community for its innovation and incredible promise in the cancer immunotherapy space.
In fact, it was their co-mentee and student, Chowdhury, who got this dream team together in the first place.
Inspired by the explosion of cancer immunotherapy studies, Chowdhury, a PhD student in the Integrated Program at Columbia’s Vagelos College of Physicians & Surgeons, began brainstorming ways to couple his interest in synthetic biology with cancer immunotherapy. At the time, Chowdhury had been rotating in the Danino lab, and Dr. Danino, associate professor of biomedical engineering and member of the Herbert Irving Comprehensive Cancer Center (HICCC), had been investigating strains of bacteria for a programmable delivery system to kill cancer cells.

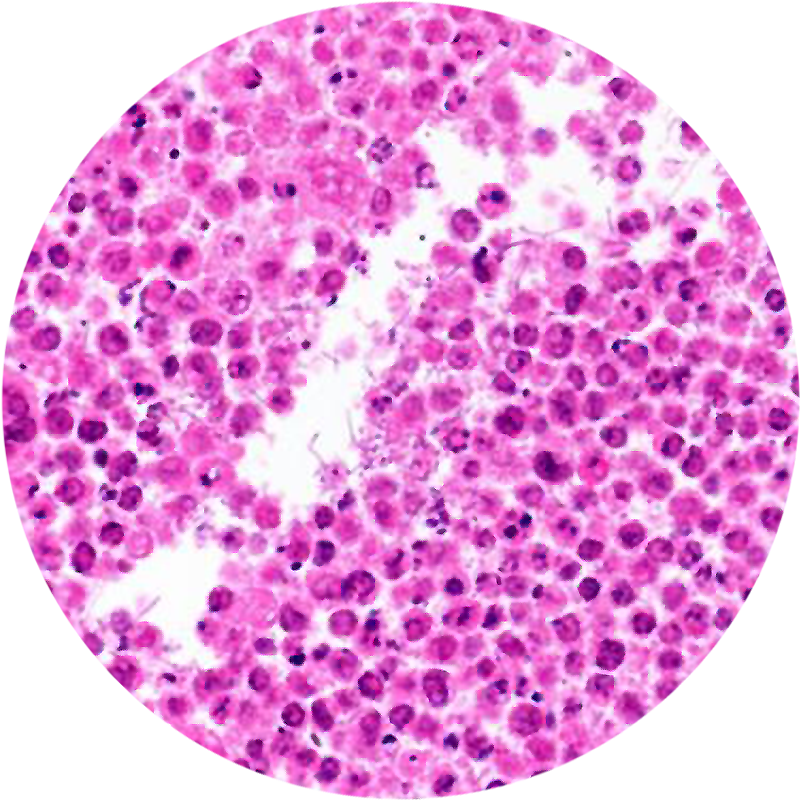

Histological section of a lymphoma tumor removed from a mouse showing tumor cells (round, pink) and bacteria (tiny rod-shaped objects in the white space).
Center Graphic:
A plasmid map of ‘pSC01’ which encodes the synchronized lysis circuit that enables bacteria to lyse and release the researchers’ engineered immunotherapeutic payload within the tumor.
Bottom Left Photo:
Sreyan Chowdhury (left), Tal Danino, PhD (middle), and Nick Arpaia (right) PhD.

CONNECTING THE DOTS IN CANCER
ENGINEERING + CANCER
“I wondered whether we could use these programmable bacteria to home to tumors, grow, and release waves of potent immunotherapeutics exclusively within the cores of tumors,” says Chowdhury. “The hope was that we could sustainably deliver high-dose immunotherapy within the tumor, while preventing off-target toxicity and side effects. For this, Tal and I both knew we needed an expert in immunology.”
In the Nature Medicine paper, the researchers demonstrated a novel engineered system to deliver immunotherapy from bacteria, which is priming the patient’s own immune system to fight the cancer. In a mouse model of lymphoma, the therapy led not only to complete regression of treated tumors, but also demonstrated it could prime the immune system to seek and treat distant untreated tumors.
The team recently developed engineered probiotics to safely deliver immunotherapies within tumors, work that was published February 2020 in Science Translational Medicine. These include nanobodies against two proven therapeutic targets—PD-L1 and CTLA-4. Through this platform, drugs are continuously released by bacteria and continue to attack the tumor after just one dose, facilitating an immune response that ultimately results in tumor regression. The versatile probiotic system, say the researchers, can also be used to deliver multiple immunotherapies simultaneously, enabling the release of effective therapeutic combinations within the tumor for more difficult-to-treat cancers like colorectal cancer.
Trust plays a big role.
The beauty of Columbia’s Integrated Program in Cellular, Molecular, and Biomedical Studies (CMBS), is that it allows for graduate students in their first year to rotate in any Columbia lab involved in biomedicine broadly, and that’s precisely what Chowdhury did. A module of the course was taught by Dr. Arpaia, assistant professor of microbiology and immunology and member of the HICCC. Chowdhury began learning more about Dr. Arpaia’s research, including his graduate work in understanding immune factors governing virulence of pathogens and his postdoctoral work on the diverse roles of regulatory T cells in health and disease.
“Inspired by Nick’s papers and lectures in immunology, I brought up the idea of setting up a meeting between the three of us. Tal is super supportive of his trainees forging new research directions for the lab so he instantly agreed,” says Chowdhury.
The three have since formed a tightly knit research team, with their eyes set on cutting-edge cancer research. Both Drs. Arpaia and Danino lean to out-of-the-box ideas that meld bacterial cancer therapy and immunotherapy in a way to increase its efficacy as a therapeutic and also enhance its safety.
© Columbia University Irving Medical Center,
Herbert Irving Comprehensive Cancer Center,
New York, NY.
Credit and Cast
Download Print-friendly
HICCC Annual Report 2020

Programmed Bacteria for Novel Cancer Immunotherapy
“I don’t know everything Tal knows about synthetic biology, and I’m not going to. And Tal isn’t going to know everything I know about immunology,” says Dr. Arpaia. “Our collaboration is us agreeably trusting each other for our own expertise. And, we’re jointly making sure that we have our bases covered but also explaining to each other the cool parts about our fields.”
The team is working on optimizing their bacteria-based cancer immunotherapy to eventually test in the clinic, and have had conversations about therapeutic dosing and toxicity with Gary Schwartz, MD, deputy director of the HICCC, an oncologist, and visionary in targeted cancer therapy. At the end of the day, adds Dr. Arpaia, “We are all equally committed to doing impactful science the best way we can.”
“I’m so happy that Nick and Tal are writing and winning multiple grants together and involving more members of both labs on collaborative projects,” says Chowdhury. “They’re pretty close friends now, and I’m happy to have been the catalyst.”
Programmed Bacteria for Novel Cancer Immunotherapy



