
IN THE FACE OF A PANDEMIC
HICCC Researchers Address COVID-19
Cracking Down on COVID-19
Faces of the Frontline
CONNECTING THE DOTS IN CANCER
Engineering + Cancer
Dentistry + Cancer
Public Health + Cancer
RESEARCHERS ON THE CUTTING EDGE
Hitting Pancreatic Cancer Where it Hurts
Commanding Killer T Cells
Election to National Academies
MAKING A DIFFERENCE
A “CURE” for Budding Scientists
Newfound Hope with Cell TherapyVelocity Goes Virtual
Unicorns in the Treatment Rooms
ACKNOWLEDGMENTS
CONTACT US

IN THE FACE OF A PANDEMIC
HICCC Researchers Address COVID-19
Cracking Down on COVID-19
Faces of the Frontline
CONNECTING THE DOTS IN CANCER
Engineering + Cancer
Dentistry + Cancer
Public Health + Cancer
RESEARCHERS ON THE CUTTING EDGE
Hitting Pancreatic Cancer Where it Hurts
Commanding Killer T Cells
Election to National Academies
MAKING A DIFFERENCE
A “CURE” for Budding Scientists
Newfound Hope with Cell TherapyVelocity Goes Virtual
Unicorns in the Treatment Rooms
ACKNOWLEDGMENTS
CONTACT US
RESEARCHERS ON THE
CUTTING EDGE
CUTTING EDGE
COMMANDING KILLER T CELLS

Dr. Donna Farber's investigations of these tissue-resident T cells have larger implications for treating cancer with immunotherapy as well as better understanding diseases like COVID-19.
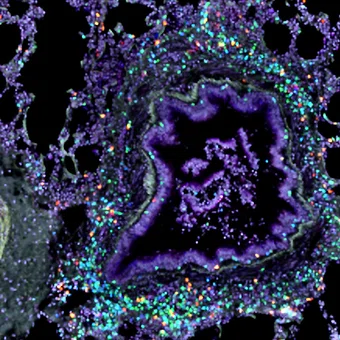
Confocal image of the lung showing staining of tissue resident memory T cells surrounding a major airway.
While immune cells are studied most often through blood samples, sampling immune cells in blood does not include the many types of non-circulating immune cells based in tissues. In fact, most of the body’s lymphocytes—the type of white blood cell that includes T cells—take up residence in places like the lungs, intestines, liver, and skin rather than in the bloodstream.
Previously, Dr. Farber and her colleagues discovered a new subset of tissue-resident T cells that appeared in the lungs of mice after influenza infection. These virus-specific T cells killed all the infected cells in the lung, clearing out the infection—and unexpectedly stuck around afterward.
“The T cells stayed exactly where they needed to be, around the airways and just remained there like sentries. When re-challenged with the virus, the mice were fully protected, and the virus was cleared very quickly,” she says. “In other words, you have your immune response exactly where you need it, and it’s all ready for action.”
To go beyond animal studies, Dr. Farber and her colleagues established a first-of-its-kind collaboration with the organ procurement organization for the New York Metropolitan area, LiveOnNY, to obtain healthy human tissue samples for research on the human immune system. If an individual has consented to have any tissue or organs used for research, a transplant surgeon from NewYork-Presbyterian Hospitals will go to the site of acquisition to gather the donated samples and bring them back to Columbia. Other research institutions around the world have since adopted this same type of arrangement.
This approach also allows the Farber lab to study multiple tissues from a single person to produce maps of how the immune system is organized throughout the body. These maps generate a new baseline for a healthy immune response in tissue, which can be used to understand how that situation changes when it comes to cancer.
When the pandemic hit New York City in March, Dr. Farber decided to pivot her research and use her expertise in immunology to study COVID-19. Her laboratory analyzed respiratory secretions of patients with COVID-19, which contain tissue-resident T cells, and compared them to blood samples.
“What we found correlated with survival was what was happening in the airway, not in the blood. So more T cells in the airway correlated with a better outcome, whereas the proportion of T cells in the blood didn’t correlate,” says Dr. Farber, who recently received a grant from the Chan Zuckerberg Initiative for this line of research.
In addition, she is applying her knowledge of how the immune system changes with age to another COVID-19 puzzle. Children are often asymptomatic, while older adults remain at risk for developing serious complications. Why does this large discrepancy exist? Dr. Farber says one reason is that the number of naïve T cells, which learn to recognize new pathogens, declines sharply with age.
“Older people really don’t have many naïve T cells left, and that is a known phenomenon, but we were able to show that you lose them in tissues as well,” she says. “Normally the lack of naïve T cells in adults does not affect responses to pathogens that we regularly encounter because adults have already established efficient immunological memory responses and one rarely encounters pathogens that are completely new, like SARS-CoV-2. Children, however, have ample supplies of naïve T cells to respond to newly encountered pathogens and therefore are faring better overall.”
Despite being a type of white blood cell, the T cells that immunologist Donna Farber, PhD, studies aren’t found in the bloodstream. Instead, these immune cells remain stationed in the lungs and other organs like soldiers, ready to attack invading pathogens, including tumor cells.
Her investigations of these tissue-resident T cells, as they’re called, have larger implications for treating cancer with immunotherapy as well as better understanding diseases like COVID-19.
“We typically think of immune cells in humans as being in blood, but in fact, the majority of your immune cells are in tissues,” says Dr. Farber, a member of the Herbert Irving Comprehensive Cancer Center and professor of surgical sciences and of microbiology & immunology at Columbia’s Vagelos College of Physicians and Surgeons. “Cancer will tend to arise within tissue, and there’s a growing realization that these tissue-resident T cells are playing a role in anti-tumor immunity at the site.”
His laboratory works on finding new strategies for treatment that target aspects of biology needed by pancreatic cancer to survive. In other words, Dr. Olive and his colleagues look for an Achilles’ heel—called a targetable critical dependency—and see how well a certain drug or combination of drugs works to exploit that weakness.
Recently, they discovered that a drug in development for a rare kidney stone disease acts on one of these targetable critical dependencies for pancreatic cancer. Cancer cells have a detoxification system that allows them to get rid of reactive oxygen species (ROS), which left uncontrolled, will wreak havoc on DNA and key proteins. When tumors grow, they create an excess of ROS, and it needs to be cleared by the detoxification system.
The researchers wondered if dismantling this detoxification system and letting ROS run wild in tumor cells would prove effective against pancreatic cancer. They decided to inhibit a particular transporter that brings an amino acid called cysteine — a key resource needed for the detox process—inside the cell. To test their hunch, Dr. Olive and his colleagues genetically engineered mice in which the transporter could be suddenly deleted and found that this caused their tumors to stop growing or regress.
“Of course, we’re not going to genetically engineer patients, so we needed a drug to do the same thing,” he says. “We encountered a paper reporting on an artificial enzyme that could break down cysteine in the blood. We thought, this seems appropriate—fortuitous, in fact.”
They obtained the enzyme, which is being developed for the treatment of cystinuria, a genetic disorder that leads to high levels of cysteine in the urine. When given to mice with pancreatic tumors, the cancer cells began to die. This work, helmed by Dr. Olive’s former graduate student Michael A. Badgley, PhD, was published by Science in April.
Commanding
Killer T Cells
Tissue-resident T Cells Act as Sentries, Ready to Attack Invading Pathogens
Cover photo:
Donna Farber, PhD
RESEARCHERS ON THE CUTTING EDGE
Commanding Killer
T Cells
Commanding Killer T Cells
Tissue-resident T Cells Act as Sentries, Ready to Attack Invading Pathogens
Despite being a type of white blood cell, the T cells that immunologist Donna Farber, PhD, studies aren’t found in the bloodstream. Instead, these immune cells remain stationed in the lungs and other organs like soldiers, ready to attack invading pathogens, including tumor cells.
Her investigations of these tissue-resident T cells, as they’re called, have larger implications for treating cancer with immunotherapy as well as better understanding diseases like COVID-19.
“We typically think of immune cells in humans as being in blood, but in fact, the majority of your immune cells are in tissues,” says Dr. Farber, a member of the Herbert Irving Comprehensive Cancer Center and professor of surgical sciences and of microbiology & immunology at Columbia’s Vagelos College of Physicians and Surgeons. “Cancer will tend to arise within tissue, and there’s a growing realization that these tissue-resident T cells are playing a role in anti-tumor immunity at the site.”

Donna Farber, PhD
Dr. Donna Farber's investigations of these tissue-resident T cells have larger implications for treating cancer with immunotherapy as well as better understanding diseases like COVID-19.
Confocal image of the lung showing staining of tissue resident memory T cells surrounding a major airway.
“What we found correlated with survival was what was happening in the airway, not in the blood. So more T cells in the airway correlated with a better outcome, whereas the proportion of T cells in the blood didn’t correlate,” says Dr. Farber, who recently received a grant from the Chan Zuckerberg Initiative for this line of research.
In addition, she is applying her knowledge of how the immune system changes with age to another COVID-19 puzzle. Children are often asymptomatic, while older adults remain at risk for developing serious complications. Why does this large discrepancy exist? Dr. Farber says one reason is that the number of naïve T cells, which learn to recognize new pathogens, declines sharply with age.
“Older people really don’t have many naïve T cells left, and that is a known phenomenon, but we were able to show that you lose them in tissues as well,” she says. “Normally the lack of naïve T cells in adults does not affect responses to pathogens that we regularly encounter because adults have already established efficient immunological memory responses and one rarely encounters pathogens that are completely new, like SARS-CoV-2. Children, however, have ample supplies of naïve T cells to respond to newly encountered pathogens and therefore are faring better overall.”
While immune cells are studied most often through blood samples, sampling immune cells in blood does not include the many types of non-circulating immune cells based in tissues. In fact, most of the body’s lymphocytes—the type of white blood cell that includes T cells—take up residence in places like the lungs, intestines, liver, and skin rather than in the bloodstream.
Previously, Dr. Farber and her colleagues discovered a new subset of tissue-resident T cells that appeared in the lungs of mice after influenza infection. These virus-specific T cells killed all the infected cells in the lung, clearing out the infection—and unexpectedly stuck around afterward.
“The T cells stayed exactly where they needed to be, around the airways and just remained there like sentries. When re-challenged with the virus, the mice were fully protected, and the virus was cleared very quickly,” she says. “In other words, you have your immune response exactly where you need it, and it’s all ready for action.”
To go beyond animal studies, Dr. Farber and her colleagues established a first-of-its-kind collaboration with the organ procurement organization for the New York Metropolitan area, LiveOnNY, to obtain healthy human tissue samples for research on the human immune system. If an individual has consented to have any tissue or organs used for research, a transplant surgeon from NewYork-Presbyterian Hospitals will go to the site of acquisition to gather the donated samples and bring them back to Columbia. Other research institutions around the world have since adopted this same type of arrangement.
This approach also allows the Farber lab to study multiple tissues from a single person to produce maps of how the immune system is organized throughout the body. These maps generate a new baseline for a healthy immune response in tissue, which can be used to understand how that situation changes when it comes to cancer.
When the pandemic hit New York City in March, Dr. Farber decided to pivot her research and use her expertise in immunology to study COVID-19. Her laboratory analyzed respiratory secretions of patients with COVID-19, which contain tissue-resident T cells, and compared them to blood samples.
© Columbia University Irving Medical Center,
Herbert Irving Comprehensive Cancer Center,
New York, NY.
Credit and Cast
Download Print-friendly
HICCC Annual Report 2020

